Synthetic Chemistry

Streptothricin F was discovered over 70 years ago by Waksman and Woodruff and displays an impressive activity spectrum against otherwise resistant Gram-negative pathogens. Initial concerns around toxicities and the availability of alternative antibiotics precluded its development. There is one reported streptothricin F total synthesis published in 1982 by Kusumoto. This original synthesis is largely linear and does not lend its hand to medicinal chemistry approaches. We have developed a novel total synthesis that is highly tunable for medicinal chemistry. Through a divergent total synthetic approach, we will pursue SAR exploration of the three primary molecular constituents of this natural product.

Xanthommatin is a member of the ommatin class; natural colorants found in cephalopods and arthropods. As a pigment, it changes color from yellow (oxidized) to red (reduced). While there have been two syntheses of xanthommatin in the past, they are either low yielding or require expensive catalysts and starting materials. We developed a scalable seven-step bio-inspired synthesis of this molecule, and are currently pursuing methodology development using xanthommatin as a novel electrochemical catalyst for oxidations.
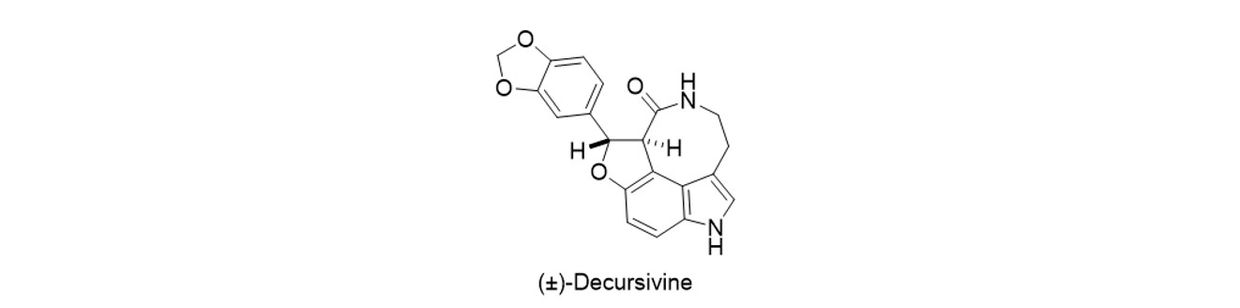
(±)-Decursivine is an indole alkaloid that is isolated in optically active form from the leaves and stems of Rhaphidophora decursiva Schott (Araceae). Decursivine contains a unique tetracyclic framework consisting of a trans-dihydrobenzofuran, an indole, and an eight-membered lactam that bridges the indole 3- and 4-positions. Decursivine exhibits antimalarial activity against the D6 and W2 isolates of Plasmodium falciparumwith IC50 values of 3.93 and 4.41 µg/mL respectively. Our synthesis of Decursivine represents one of the shortest and highest yielding synthesis of this natural product to date.
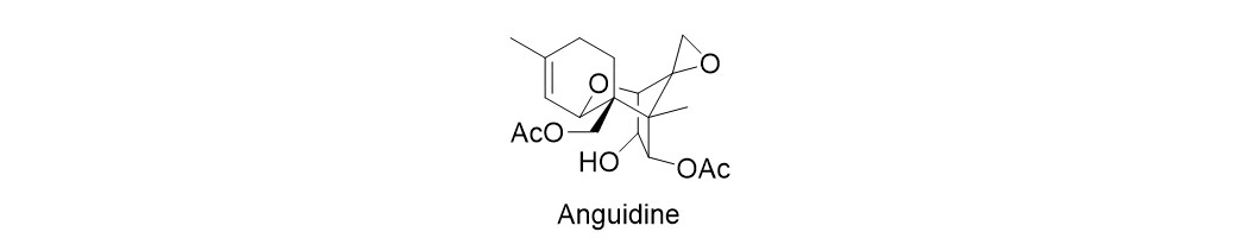
Anguidine and other related trichothecenes have been actively pursued due to their broad biological activities that directly correlate to the respective functional groups on the tricyclic backbone. Anguidine itself has been shown to inhibit the initiation of protein synthesis resulting in the death of rapidly proliferating cells such as tumors and has shown good antibacterial, antifungal and insecticidal activity. Various studies to develop an enantioselective synthesis of anguidine and anguidine precursors have been previously reported. We aim to develop a practical and scalable anguidine synthesis that enables the preparation of the anguidine scaffold with various ring sizes in racemic and enantiopure form.
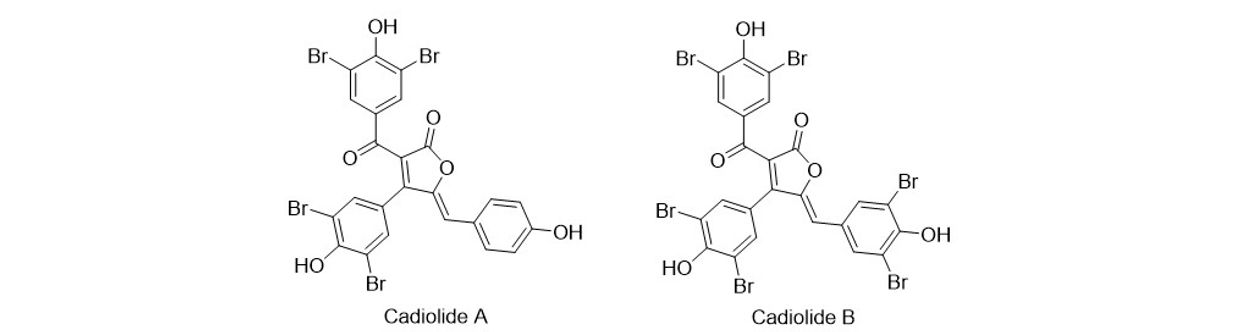
Cadiolides A and B are secondary metabolites from marine ascidians and tunicates, and they were found to have potent activity against Gram positive bacteria like Methycillin Resistance Staphylococcus Aureus (MRSA).
The Manetsch Laboratory is also interested in protocol development to aide in the discovery and optimization of chemical reactions enabling the straightforward preparation of compounds with interesting activities or properties. For our endeavors in hit-to-lead optimizations, kinetic target-guided synthesis, and the preparation of probe molecules,the development of new synthetic protocols has been instrumental.
Below are selected publications that highlight some of the synthetic protocols that the Manetsch laboratory has developed.
Select Publications

The convergent total synthesis and antibacterial profile of the natural product streptothricin f.
Dowgiallo, M.; Miller, B.; Kassu, M.; Smith, K. P.; Fetigan, A.; Guo, J.; Kirby, J.; Manetsch, R. Chem. Sci. 2022.

Total Synthesis of (±)-Decursivine via BINOL-Phosphoric Acid Catalyzed Tandem Oxidative Cyclization.
Parvatkar, P. T.; Smotkin, E. S.; Manetsch, R. Sci Rep 2021.
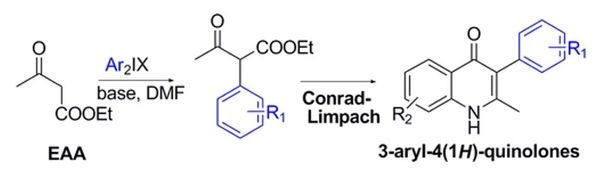
Monastyrskyi A, Namelikonda N K, Manetsch R. J Org Chem 2014; 80, 2513-2502
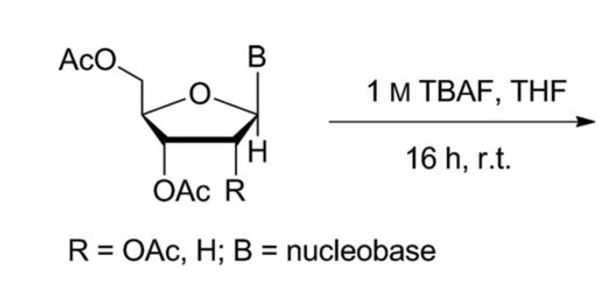
Kumar, A. B.; Manetsch, R. European J. Org. Chem. 2014, 3551–3555.
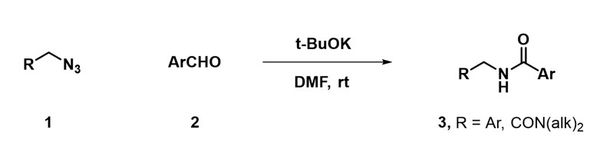
A simple base-mediated amidation of aldehydes with azides.
Kulkarni, S. S.; Hu, X.; Manetsch, R. Chem. Commun. (Camb). 2013, 49, 1193–1195.
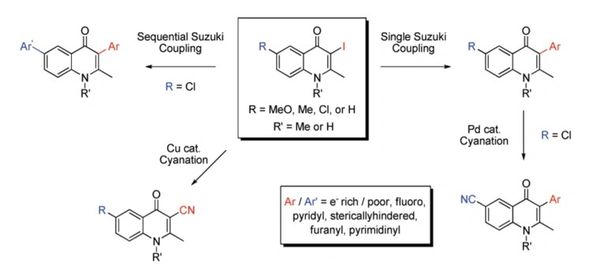
Divergent route to access structurally diverse 4-quinolones via mono or sequential cross-couplings.
Cross, R. M.; Manetsch, R. J. Org. Chem. 2010, 75, 8654–8657.