Hit-to-Lead Optimization
The process of hit-to-lead optimization in the Manetsch laboratory focuses on the use of synthetic chemistry in close conjunction with liquid chromatography coupled to mass spectrometry (LC/MS). Structure-activity relationship (SAR) studies, in parallel with structure-property relationship (SPR) studies guide the direction of our synthesis, while various LC/MS-based assays are routinely utilized for the assessment of key physicochemical properties in order to determine and identify property liabilities, which critically affect the outcome of in vivo efficacy studies.
Our laboratory applies hit-to-lead optimization to the research of the following diseases:
Neglected tropical diseases: Malaria and leishmaniasis
Bacterial diseases: Methicillin-resistant Staphylococcus aureus (MRSA), Acinetobacter baumannii, and carbapenem-resistant Enterobacteriacea (CRE)
Malaria Highlight
In 2018, the World Health Organization reported an estimate of 219 million malaria cases, with approximately 435,000 deaths in 2017. Malaria transmission is caused by the genus, Plasmodium, where the two most prevalent species responsible for this disease in human are Plasmodium vivax and Plasmodium falciparum. Due to the ever-increasing rate of resistance, a new drug class is in urgent need to treat all stages of infection. This resistance is due to the mutation in cytochrome bc1 complex, which is responsible for the electron transfer chemistry that ultimately produces adenosine triphosphate (ATP). This complex is made up of 3 subunits: cytochrome b, cytochrome c1, and the iron-sulfur protein (ISP). The binding sites of this complex is in cytochrome b and are called the quinol oxidation site (Qo) and the quinone reduction site (Qi), where Qo site is susceptible to mutation. For approximately half a century, endochin and analogues thereof were known to be causal prophylactic and potent erythrocytic stage agents in avian but not in mammalian malaria models.
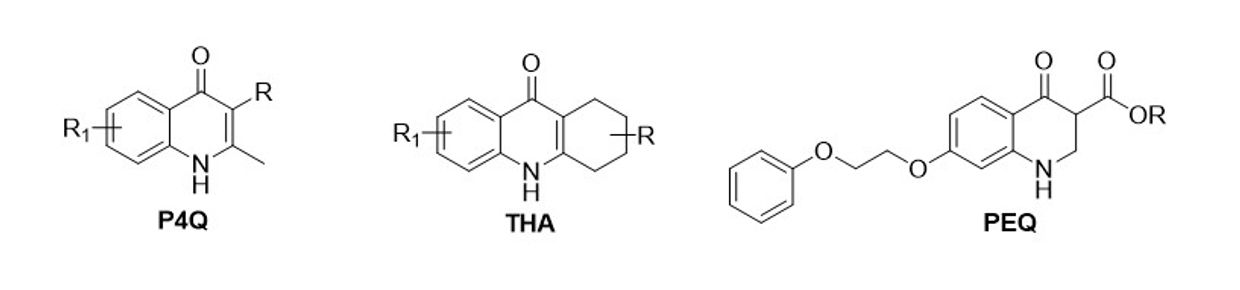
Our hit-to-lead optimization efforts lead to orally bioavailable 4(1H)-quinolones with antimalarial activity against erythrocytic and exoerythrocytic stages. Further refinement has directed our current synthesis efforts towards producing orally bioavailable 1,2,3,4-tetrahydroacridin-9(10H)-one (THA) analogs, 3-alkyl- or 3-phenyl-4(1H)-quinolone (P4Q) analogs, and 7-(2-phenoxyethoxy)-4(1H)-quinolone (PEQ) analogs.